Research Projects –
Investigating Trikafta
during pregnancy
Investigating Trikafta during pregnancy
Project Duration – 1 Year
Dr Elena Schneider-Futschik and her team at the University of Melbourne are exploring the safety and effectiveness of Trikafta in pregnancy on mothers and their babies.
With Cure4CF’s funding, Dr Schneider-Futschik’s team will:
- Develop a sensitive and reproducible analytical method to measuring the components of Trikafta.
- Determine the amount and impact of the medicine that reaches mothers and babies during pregnancy.
The team will explore this in the babies of mothers who have CF and in CF babies whose mothers do not have CF.
Why study Trikafta during pregnancy?
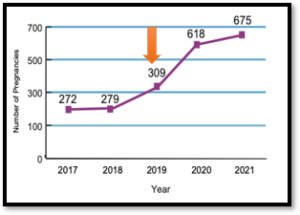 The approval of modulator drugs such as Trikafta has been a breakthrough for cystic fibrosis (CF). However, therapies such as Trikafta have not been tested for safety in pregnancy. Data also shows that the number of pregnancies in women with CF has dramatically increased in the last four years. The featured graph shows data from the USA on the number of pregnancies since 2017 and the orange arrow denotes when Trikafta was approved in the USA for people over the age of 12.
The approval of modulator drugs such as Trikafta has been a breakthrough for cystic fibrosis (CF). However, therapies such as Trikafta have not been tested for safety in pregnancy. Data also shows that the number of pregnancies in women with CF has dramatically increased in the last four years. The featured graph shows data from the USA on the number of pregnancies since 2017 and the orange arrow denotes when Trikafta was approved in the USA for people over the age of 12.
There are some small studies which suggest that Trikafta is safe during pregnancy, but there is currently no high level evidence to support women in their pregnancy planning. We now need the evidence to guide patients’ choices regarding safe use of modulator therapy in pregnancy.
What is unique about the Trikafta in pregnancy study?
Dr Schneider-Futschik has developed a sophisticated and sensitive method to detect the individual components of Trikafta in human samples. This method now requires careful validation and assessment in patient samples. Following the validation, the team will then be able to measure and understand the levels of Trikafta during pregnancy for both the mother and baby. By studying this in mothers with and without CF and their babies, the team will be able to understand how Trikafta is absorbed and moves across the placenta during pregnancy and what the CF specific implications are for both mother and baby. The team will seek samples from Australian patients as well as accessing samples from the Mayflowers trial, a USA study evaluating the maternal and foetal outcomes during pregnancy in women enrolled in the CF Foundation Patient Registry being led by Professor Taylor-Cousar.
How will this research aim to improve lives of people with CF?
Early data suggests in some babies the impact is positive and that some of the early life complications are reduced or slowed. Through this work the team hope to generate data to understand if Trikafta can positively impact CF babies prior to birth by slowing or reducing CF complications.
About the team
Dr Elena Schneider-Futschik is a specialist in CF. As a National Health and Medical Research Council (NHMRC) Research Fellow, she leads the cystic fibrosis pharmacology research program across the department “Biochemistry & Pharmacology” at the University of Melbourne aiming to optimise currently available treatments. She has received multiple awards and research support from both public and philanthropic organisations including the Thoracic Society of Australia and New Zealand, the Australian CF Trust, the Cystic Fibrosis Foundation and the NHMRC.
Professor Peter Middleton is a Respiratory & Sleep Medicine Specialist and Director of the CF and Bronchiectasis Services at Westmead Hospital, Sydney. He has been extensively involved in the clinical trials for elexacaftor + tezacaftor + ivacaftor and was first author in the landmark NEJM paper in 2019. Before Trikafta, he led the combined ERS TSANZ task force on the care of women with airways diseases during pregnancy. He is Chair of the Australian Bronchiectasis Network, member of the ECFS Standards of Care Committee and serves on numerous Committees including the CF Foundation Data Safety and Monitoring Committee.
Professor Jennifer L. Taylor-Cousar oversees the care of children and adults with CF. Jennifer is also co-director of the Adult CF Program and director of the CF Therapeutics Development Network research that is conducted at National Jewish Health. Jennifer is the chair of the Cystic Fibrosis Foundation’s Women’s Health Research Working Group.

Dr Elena Schneider-Futschik
The University of Melbourne

Professor Peter Middleton
Westmead Hospital, Sydney

Professor Jennifer L. Taylor-Cousar
National Jewish Health (Colorado, US)
