Cure4CF Update
Gene therapy update – how close are we?
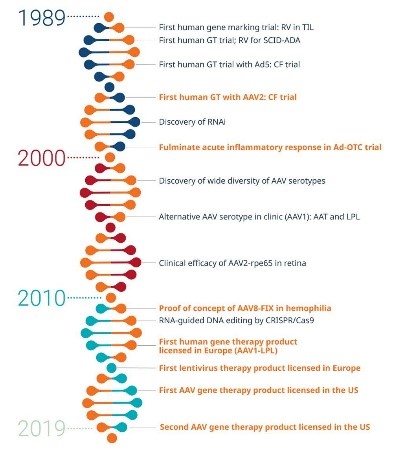
The first human genes were cloned in the 1970s with early studies commencing in the 1980s. The schematic on the right shows the progress in gene therapy over the last 3 decades. As you can see in the last 20 years progress has been rapidly escalating. Another way to show the progress is to consider the number of scientific papers have been published in gene therapy. From 1979 to 1999 around 36 000 scientific papers were published on the topic. This number increased dramatically between 1999 and 2019 when there have been more than 340,000 scientific papers published. In the last 2 years alone there have been more than 50,000 scientific papers published that involve gene therapy.
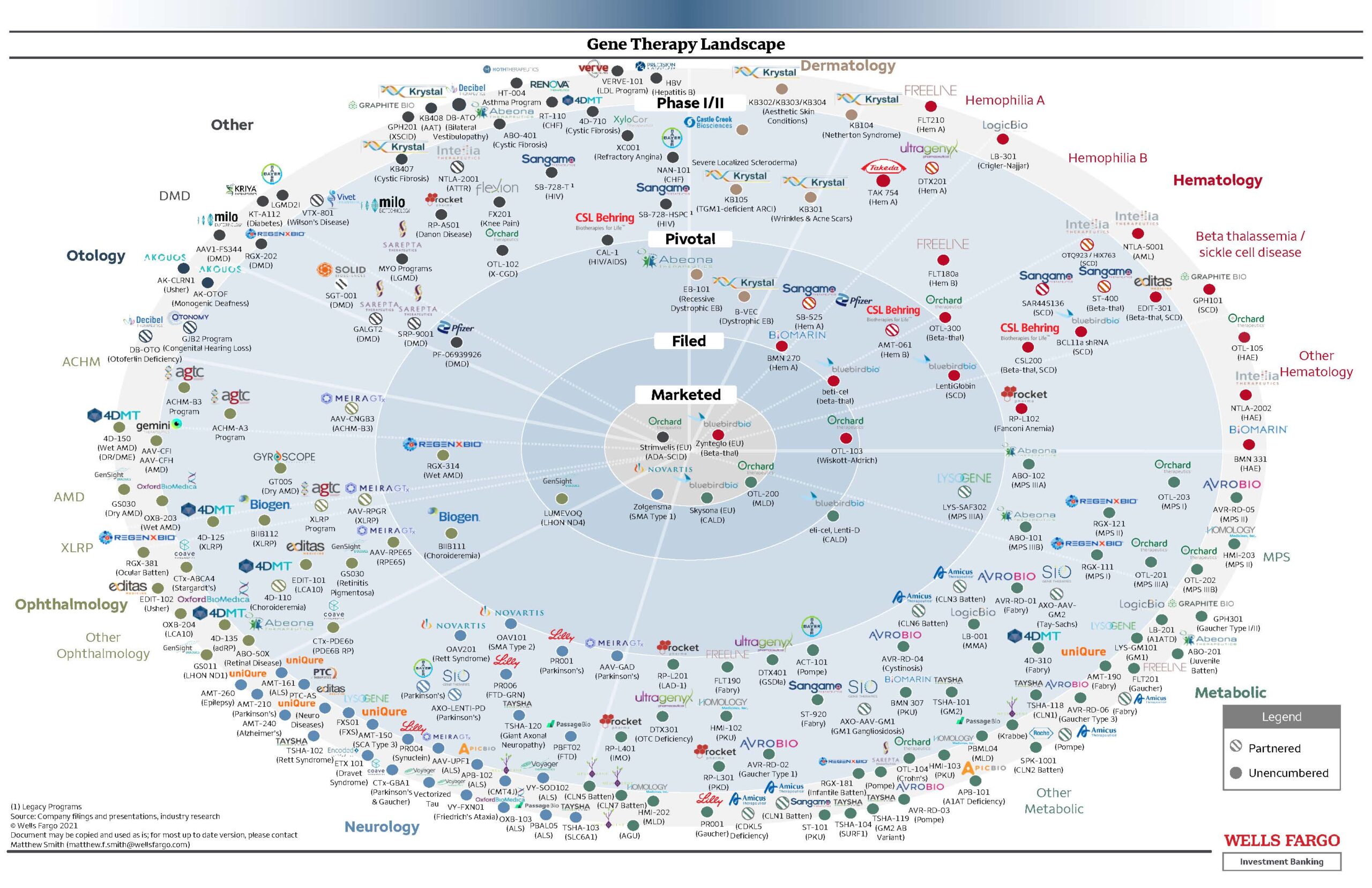
The bullseye picture below shows the gene therapy landscape and the pathway from pre-clinical to marketed products. It is a long and expensive process to generate the ideas, test them in the laboratory and then translate those ideas into clinical trials in people. It is quite an overwhelming picture. Each dot is a product being explored for different conditions and some have moved into a marketed product. This shows the large number of treatments currently under investigation for a large range of problems. One drug that has made it through the process is Zynteglo which is now available for people with beta thalassemia. This treatment involves collecting the patients blood cells and then they are modified to add a working copy of the gene before returning the modified cells to the patient using an intravenous infusion.
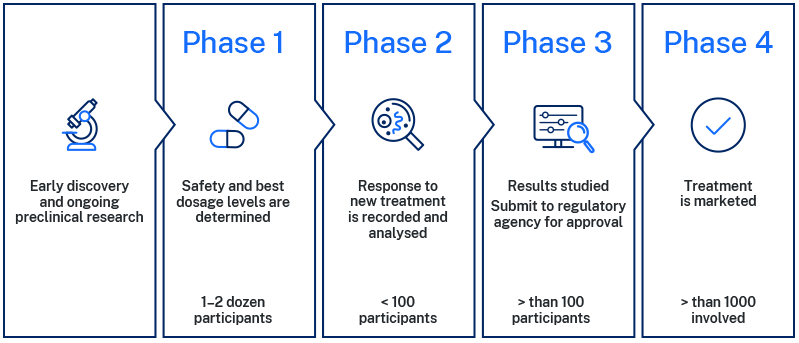
For people with cystic fibrosis, there is still more work to be done for a treatment to reach this stage of the approval and licensing stage of development. New medicines need to be tested thoroughly in early discovery and pre-clinical laboratory studies to determine if the treatment is safe and effective. This is usually undertaken using models which can be cells, animals (such as mice or rats) or combinations of both. The different phases of clinical trials are shown in the diagram below.
The first stages of clinical trials are phase 1 and 2 studies which are generally designed to establish the safety and dosage of the treatment. Once the treatment is shown to be safe in healthy people it is often then tested in a group of people with the disease of interest. The final stages are phase 3 clinical trials which involve larger numbers and carefully designed studies with a placebo control. This placebo is vital to ensure the treatment works as many people who participate in research studies find their symptoms and other outcomes improve from being monitored in the study, even without the treatment.
The Cystic Fibrosis Foundation web page has a great resource around the latest gene therapy research. There are currently two studies in phase 2 clinical trials. This means they have completed phase 1 safety studies and have progressed to studies of people with cystic fibrosis.
The first is SPL84 which is exploring a therapy for people with splicing mutations. RNA splicing is an important process in our bodies which involves cutting up and rearranging sections of RNA. When these pieces of RNA are not arranged in the correct way there can be a mutated CFTR protein produced. This study is looking at a SPL84 which can ensure that RNA is cut and rearranged correctly so that functional CFTR protein can be made. The current clinical trial is looking at the finding the right dose for patients to achieve a safe and effective treatment. It is recruiting people with the 3849 +10 Kb C->T mutation. The study is only recruiting in the USA and is hoping to enrol 24 adults. Some research suggests this mutation may be associated with a milder course of CF disease and there may be a positive response to Ivacaftor in some people. According to 2023 Australian data there are 34 people in Australia with this mutation.
The other trial in phase 2 is VX-522 mRNA, this is an inhaled messenger RNA therapy which is using a lipid nanoparticle (LNP) delivery system to deliver a full copy of CFTR mRNA to lung cells. A lipid nanoparticle is simply a lipid shell that can be used to carry medicines or in this case nucleic acids (mRNA) to our cells. The cells in our body have a lipid membrane surrounding them to protect the contents. In the image on the right, you can see that these lipids form a secure cell membrane protecting the inside of the cell from the outside.
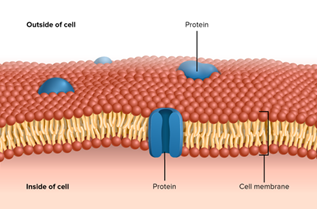
So LNP technology is a way to help the nucleic acid enter our cells. This trial is currently recruiting adults with two mutations that are not responsive to modulator therapy in the USA and is a Vertex pharmaceutical trial. Click here to read more. In Australia there are more than 300 people who might benefit from this gene therapy.
While having two trials at the phase 2 stage is very exciting, as you can see from the bullseye above, we need many more treatments being explored to ensure that some are successful in making it through to marketing and licensing. The good news is that there are also currently 4 other treatments in phase 1 clinical trials which are using different types of delivery systems including LNP and another delivery system using an adeno-associated vector (AAV) and many more in pre-clinical studies.
What is Cure4CF doing in gene therapy?
At Cure4CF we are excited to be contributing to this gene therapy pipeline in Australia with two projects in pre-clinical studies. These projects are being led by Dr Gerrard Kaiko (Newcastle) and Dr Leszek Lisowski (Sydney).
The team in Newcastle have been able to create the packages called exosomes which contain their CFTR boosting complex and have now tested this alone and in combination with current CF modulator drugs. They have investigated the delivery of the complex to lung cells in the laboratory. They are currently working on the quality control and scalability experiments needed to produce the exosomes.
The team in Sydney are exploring a strategy to edit the CFTR gene and correct CF causing mutations as well as a novel way to deliver the working gene. The team hope to use an AAV delivery system to transport a working gene to lung cells of people with CF. Now, they are working to determine the best and safest way to edit the CFTR gene and deliver this to cells. Following this, the team then need to begin to look at the right dose and delivery strategy.
Both of these programs are working hard to learn more about the best ways to design and delivery gene therapy to people with CF.
“I’m filled with hope because gene therapy won’t be about managing symptoms anymore—it’s about targeting the root cause of cystic fibrosis. For the first time, I feel like there’s a future on the horizon where we will be able to breathe freely!”
