Cure4CF Update
Aiden’s Story: How Australian Research Helped Change National Access to Life-Saving CF Treatment
Earlier this year, the Therapeutic Goods Administration (TGA) and Pharmaceutical Benefits Scheme (PBS) expanded access to Trikafta therapy, opening the door for Australians with cystic fibrosis (CF) who live with rare CFTR mutations. For the first time, Trikafta can now be accessed by anyone with CF who has at least one mutation shown to respond to the medication — either through clinical evidence or through laboratory testing of their own cells.
This is a transformational shift for people who have long been told their mutation was “too rare” for precision therapies. And it is a change made possible by dedicated researchers, clinicians, advocates, and community members who volunteered their time, their stories, and their samples.
One of those people is Aiden.
Aiden’s Journey with CF
Aiden’s early childhood was marked by unexplained illness and long periods of recovery. At just five and a half, after repeated hospital visits and renewed testing, doctors finally discovered he had cystic fibrosis — a diagnosis that had been missed at birth because he carries a rarer CFTR mutation found in only around 4% of people with CF worldwide.
Growing up, Aiden faced the challenges familiar to many in the CF community: daily treatments, physiotherapy, chronic infections, and time away from school and play. He developed Type 1 diabetes at age seven and managed early signs of liver disease, further complicating his day-to-day care.
Despite this, Aiden lived a full and active childhood, supported by a family who built routines around care, appointments, and staying well. His life changed again at age 15, when he began Kalydeco — one of the first CFTR modulators. His lung function climbed dramatically into the 120–130% range, and his hospital admissions became less frequent. Kalydeco helped for many years, but as he got older as was the case for most people on this medicine his lung function again began to decline once more.
This is where research stepped in.
Aiden’s Contribution to Research: The Origin-1 Trial
Aiden participated in the Origin-1 Trial, led by the CF research team at the University of Newcastle and the Hunter Medical Research Institute (HMRI). The trial aimed to understand how people with rarer CFTR mutations respond to Trikafta through precision medicine, using cutting-edge tests that measure how well everyone’s cells react to the therapy.
For the first time, Aiden was able to try Trikafta — and the results were immediate. His symptoms eased, his breathing felt lighter, and he needed fewer antibiotics. But when the trial ended in early 2025, he had to stop taking the medication.
Within just three days, he felt worse. His symptoms returned, and he needed to restart antibiotics. It was clear how vital the treatment had become.
The good news came shortly after:
The data from Aiden’s trial — including his own results — was used to support the PBS submission that expanded Trikafta access across Australia. His genetic variant was officially added to the list of approved mutations, meaning he could resume treatment.
Even more significantly, the TGA and PBS decision included a new pathway:
If a person’s cells respond to Trikafta in a validated laboratory test (such as those performed in the CF lab in Newcastle) and/or short term clinical trial while receiving the drug, they are now eligible to receive the medication long term — regardless of how rare their mutation is.
This is personalised medicine in action.
This is research shaping policy.
This is the CF community driving change.
Inside the CF Research Lab: Our Visit with Associate Professor Gerard Kaiko and Amber Pillar
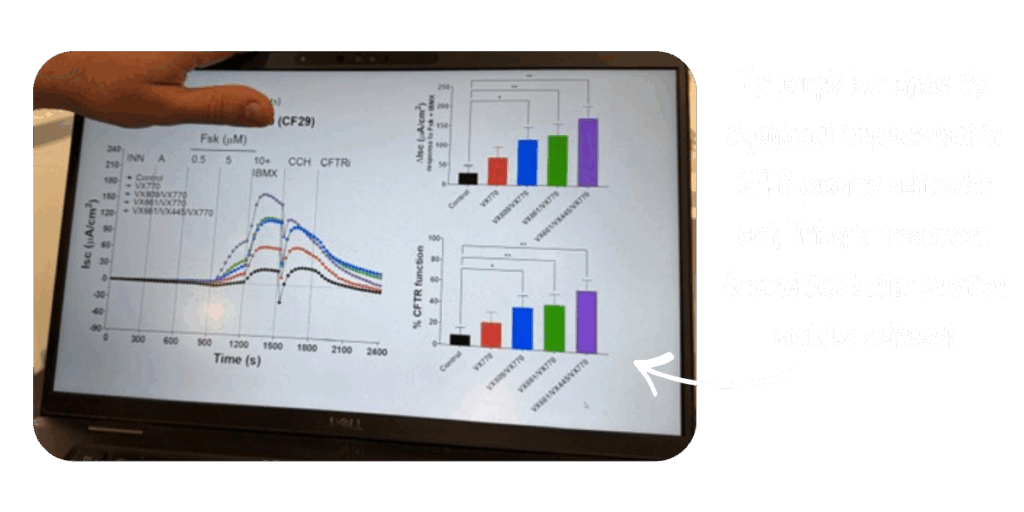
During our recent visit to the CF Research Laboratory at the Hunter Medical Research Institute, led by Associate Professor Gerard Kaiko, we saw first-hand how this ground-breaking work is done.
A/Professor Kaiko and his team are focused on understanding how Trikafta works for people with rare and currently “unclassified” CFTR mutations. Since the recent TGA/PBS decisions they are now prioritising testing for individuals with class I variants, where little or no international evidence is available or where the evidence is based only on computer predictions and not real-world lab evidence. Their work in the ongoing ORIGIN-1 trial will attempt to identify whether any of these people may respond to Trikafta.

In Aiden’s case, the testing clearly showed that Trikafta produced a stronger response than Kalydeco. Since returning to Trikafta after the TGA approval, Aiden’s lung function has improved again, and he is feeling more stable and well.
Seeing this research up close is a powerful reminder of why Cure4CF invests in high-impact science. This is Australian innovation directly improving the lives of Australians with CF.
Research in Action. Real Lives Changed.
Aiden’s story represents what so many in the CF community strive for:
Hope grounded in evidence. Progress powered by research. Lives transformed by access.
The TGA and PBS’s decision reflects the strength of global scientific collaboration — data provided by Vertex, to international studies, to the ground-breaking evidence generated in our own backyard here in Newcastle.
Aiden’s participation in research didn’t just change his life. It helped change national treatment guidelines. It opened doors for others with rare mutations. And it demonstrated the profound impact of community involvement in research.
At Cure4CF, this is why we do what we do — to accelerate discoveries, support innovative science, and ensure that every person with CF has the chance to breathe easier and live longer, fuller lives.
